Witnessing the transformation of metal through the captivating process of oxidation is like observing nature's silent dance, where time leaves its indelible mark. A symphony of colors emerges, as vibrant hues and intricate textures replace the luster of fresh steel. This metamorphosis presents a paradoxical beauty, as something deemed deteriorated becomes an emblem of resilience and character. Delving into the mesmerizing world of rust iron unveils a treasure trove of narratives, where each corroded surface holds a story waiting to be discovered.
The aging of metal, be it through exposure to the elements or deliberate human intervention, bestows upon it a unique charm that transcends its utilitarian function. As layers of corrosion take hold, a sense of history and nostalgia permeates the object. The once smooth, shiny surface is now marked by a tapestry of cracks, pits, and patterns, a testament to the passage of time and the forces of nature at play.
Exploring the world of oxidized metal is akin to embarking on an archaeological adventure, where the remnants of human endeavor intertwine with the forces of nature. As rust iron embodies a harmonious blend of strength and vulnerability, it symbolizes the transient nature of existence itself. The gentle touch of decay becomes a metaphor for the ephemeral quality of life, reminding us of the inevitability of change.
Embracing the allure of rust iron opens the door to a world filled with artistic expression and creative possibilities. It prompts us to redefine our perception of beauty, challenging the conventional notions of flawlessness, and inviting us to appreciate the imperfect and the unexpected. With each stroke of corrosion, the metal unveils a story that transcends time, making us ponder the significance of our own human journey in the face of constant transformation.
The Science Behind Corroding Metal

In this section, we will delve into the scientific principles that govern the process of metal corrosion, commonly known as rusting. We will explore the intricate mechanisms that underpin this natural oxidation process, which leads to the formation of rust on iron and other metals.
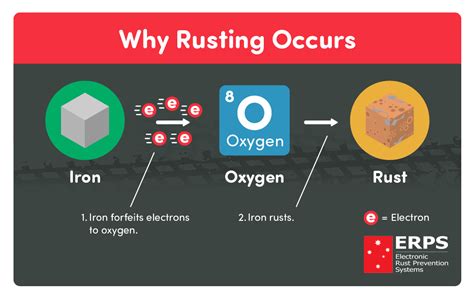
Corrosion is a complex chemical reaction that occurs when metals are exposed to oxygen and moisture over a period of time. It involves the transfer of electrons from the metal to the oxygen molecules in the presence of water, resulting in the production of metal oxide compounds.
One key factor in the rusting process is the concept of oxidation, which refers to the loss of electrons by a substance. When iron or other metal surfaces come into contact with water, they undergo an electrochemical reaction in which the metal atoms lose electrons to form positively charged ions. These ions then react with oxygen to produce metal oxide compounds, which often exhibit the familiar reddish-brown color of rust.
Understanding the various stages of rusting can provide insight into how to prevent and mitigate the damage caused by corrosion. The initial stage of rust formation involves the formation of microscopic pits on the metal surface, exposing fresh metal to the corrosive environment. These pits act as sites for further oxidation, accelerating the rusting process.
Factors such as temperature, humidity, and the presence of pollutants can significantly affect the rate of corrosion. Higher temperatures and increased moisture levels can accelerate the corrosion process, while pollutants in the environment can act as catalysts, further speeding up the formation of rust.
- Electrolytes, such as saltwater or acids, can enhance the conductivity of the corrosion process, leading to more rapid rust formation.
- Galvanic corrosion occurs when two different metals come into contact in the presence of an electrolyte, resulting in the accelerated corrosion of the less noble metal.
- Localized corrosion, such as pitting, occurs when certain areas of the metal surface experience intensified chemical reactions, leading to the formation of small holes.
By understanding the underlying science behind rusting, scientists and engineers can develop strategies to prevent or slow down the corrosion process. Protective coatings, such as paint or galvanization, can create a barrier between the metal surface and the corrosive environment. Other methods involve using corrosion inhibitors or designing alloys with improved resistance to oxidation.
Overall, the science behind rusting is a fascinating field that combines chemistry, materials science, and environmental factors. By unraveling the mysteries of rust formation, we can work towards preserving and extending the lifespan of metal structures and objects that are prone to corrosion.
The Various Forms of Oxidation
Oxidation is an intriguing chemical process that occurs when a material interacts with oxygen or other oxidizing agents, leading to various transformations and changes in its properties. In this section, we will explore the different manifestations of oxidation, delving into its diverse forms and their unique characteristics.
One of the most common types of oxidation is known as corrosion, which typically affects metals and alloys. Corrosion can result in the formation of rust, a reddish-brown substance that is synonymous with the deterioration of iron and steel. However, corrosion is not limited to just these materials. It can also affect other metals, such as copper or aluminum, producing distinctive patinas and tarnishes.
Another fascinating form of oxidation is combustion, a rapid chemical reaction that occurs when a substance combines with oxygen, releasing heat, light, and often other byproducts. Combustion can be observed in natural phenomena such as fire, where materials such as wood, paper, or fuels undergo oxidation, releasing energy in the process. Similarly, combustion powers various engines, including those found in automobiles and aircraft.
In addition to corrosion and combustion, oxidation can also manifest in processes such as oxidation-reduction reactions, commonly known as redox reactions. Redox reactions involve the transfer of electrons between different chemical species, resulting in oxidation of one substance and reduction of another. These reactions are essential in many biological and industrial processes, such as photosynthesis, respiration, and the production of various chemicals and materials.
| Type of Oxidation | Description |
|---|---|
| Corrosion | The gradual deterioration of materials, particularly metals, due to oxidation. |
| Combustion | A rapid oxidation process that releases heat, light, and other byproducts. |
| Oxidation-Reduction Reactions | Chemical reactions involving the transfer of electrons between different substances. |
As we delve further into the topic of oxidation, it becomes evident that this fascinating chemical process encompasses a wide range of transformations and phenomena. By understanding the different types of oxidation and their implications, we can gain a deeper appreciation for the role this process plays in shaping the world around us.
The Impact of Environmental Factors on the Formation of Rust
In this section, we will delve into the influence of various environmental factors on the formation of rust, an intriguing process resulting from the oxidation of iron. Understanding these factors is crucial in comprehending the complex mechanisms underlying rust formation and its effects on different materials.
1. Moisture: Moisture plays a pivotal role in the formation of rust. The presence of water or moisture in the air triggers the oxidation process, leading to the formation of iron oxide. The more humid the environment, the higher the chances of rust formation occurring at an accelerated rate.
2. Oxygen: Oxygen is another vital component necessary for the formation of rust. When iron comes into contact with oxygen, a chemical reaction occurs, resulting in the production of iron oxide, commonly known as rust. The greater the concentration of oxygen in the environment, the faster and more extensive the rust formation becomes.
3. Salinity: Salinity or the presence of salts in the environment can significantly impact rust formation. When iron is exposed to saltwater or salty air, the salt acts as a catalyst for the oxidation process, leading to a higher rate of rust formation. This is particularly visible in coastal areas or regions with high levels of salt in the air.
4. Temperature: Temperature also plays a role in the formation of rust. Higher temperatures generally increase the speed of chemical reactions, including the oxidation of iron. This means that rust formation may occur more rapidly in hot environments, compared to colder regions where the process may be slower.
5. Pollution: Environmental pollution, including pollutants such as sulfur dioxide and nitrogen oxides, can significantly impact rust formation. These pollutants can react with moisture and oxygen, creating acidic compounds that promote corrosion and enhance the rate of rust formation on iron surfaces.
6. Time: Over time, the accumulation of various environmental factors mentioned above can contribute to an increased likelihood of rust formation. The longer an iron object is exposed to moisture, oxygen, salinity, temperature fluctuations, and pollution, the greater the probability of rust developing on its surface.
Understanding how these environmental factors interact and influence rust formation is essential for developing effective preventive measures and corrosion-resistant materials. By carefully considering these factors, it is possible to minimize the impact of rust and preserve the longevity and functionality of iron objects.
The Impact of Corrosion on Various Materials and Structures
In this section, we will delve into the far-reaching consequences and effects of corrosion on a diverse range of materials and structures. Rust, a natural process resulting from the oxidation of metals, can wreak havoc on numerous objects in our everyday lives, causing significant deterioration and potential structural failures.
Corrosion, in its various forms, is a phenomenon that affects not only iron and steel but also other metals and alloys, including aluminum, copper, and bronze. The corrosive nature of rust extends beyond the realm of metal objects to impact the durability and integrity of concrete, wood, and even certain plastic materials. It is essential to understand how these different materials and structures are affected by rust to develop effective prevention and maintenance strategies.
Metal Structures
While iron and steel are most commonly associated with rust and corrosion, other metals are not exempt from its effects. Aluminum, for instance, is particularly vulnerable to corrosion, which can compromise its structural strength and overall performance. Similarly, copper is prone to corrosion, resulting in the formation of greenish deposits and jeopardizing its functionality.
Non-Metallic Materials
It is a misconception that rust only affects metals. Concrete, a widely-used construction material, is also susceptible to corrosion. Rust build-up within reinforced concrete structures can cause severe damage, leading to cracks and weakening of the structure. Wood, another common material, can undergo a form of corrosion known as fungal decay, which can significantly diminish its structural integrity.
Composite Materials
Even composite materials, which are engineered to combine the strengths of different substances, are not immune to rust's impact. Composite materials that incorporate metal components are susceptible to corrosion, which can compromise the overall strength and performance of these hybrid materials.
Conclusion
Understanding the impact of rust on different materials and structures is crucial for engineers, architects, and maintenance professionals. By comprehending the diverse ways corrosion can occur and the specific vulnerabilities of various materials, we can develop targeted strategies to prevent and mitigate rust's detrimental effects. Through research and innovation, we aim to prolong the longevity of our creations and preserve the functionality and aesthetics of our built environments.
Historical Significance of Rust and its Beauty in Art

Discovering the historical significance of rust and its captivating presence in the world of art brings forth a deeper understanding of its allure and relevance. This section aims to explore the profound impact of rust and its aesthetic appeal, transcending time and inspiring artistic expression.
Glimpses into the past reveal rust as a timeless symbol of transformation and decay, embodying the passage of time and the inevitable changes that occur. Since ancient times, rust has fascinated artists and art enthusiasts alike, evoking a sense of nostalgia and contemplation.
The rich hues and intricate patterns formed as iron reacts with oxygen have inspired artists throughout history to incorporate rust into their works. From delicate paintings showcasing the ethereal beauty of rusted metal to sculptures exuding a raw and rugged elegance, the art world has embraced the captivating qualities of rust as a medium of expression.
Furthermore, the inclusion of rust in art not only serves as a visual element but also carries symbolic significance. Rust can represent the passage of time, the fragility of existence, and the gradual erosion of human creations.
Over the centuries, renowned artists have utilized rust as a metaphor for the impermanence of life and the inevitable decay of all things. This duality of beauty and decay has created a unique dialogue between the artist, the artwork, and the viewer.
Exploring the historical significance of rust in art allows us to appreciate and celebrate the beauty found in the natural processes of oxidation and decay. It encourages us to view rust not as a mere byproduct of corrosion, but as a symbol of ephemerality and a testament to the transitory nature of existence.
Preventing and Treating Corrosion in Everyday Life
In this section, we will explore effective methods for safeguarding and addressing the issue of corrosion, a common problem encountered in our daily lives. The presence of corrosion can cause significant damage to various objects and surfaces, but by implementing preventative measures and utilizing appropriate treatments, we can extend the longevity and functionality of our belongings.
Preventative Measures:
One essential approach to preventing corrosion is to create a protective barrier between the object and its environment. This can be accomplished through the application of appropriate coatings, such as protective paint or varnish. Additionally, using corrosion-resistant materials, such as stainless steel or galvanized metals, can significantly reduce the risk of oxidation.
Another effective preventative measure is proper maintenance and regular cleaning. By keeping surfaces clean and free from moisture or contaminants, we can hinder the corrosion process and maintain the integrity of the object. Regular inspection is also crucial to identify potential areas of concern and take proactive measures before corrosion sets in.
Treatment Options:
If corrosion has already occurred, various treatment options are available to minimize further damage and restore the affected object. One commonly used method is mechanical removal, which involves scrubbing or sanding the corroded surface to eliminate rust particles. Chemical treatments, such as rust converters or inhibitors, can also be applied to convert the rust into a more stable compound or inhibit the corrosion process.
For more severe cases of corrosion, restoration professionals may employ techniques like electrochemical methods, including electrolysis or electroplating, to reestablish the object's original condition. These methods can remove rust and create a protective layer to prevent future corrosion.
By understanding the importance of preventative measures and the various treatment options available, we can actively combat the effects of corrosion in our everyday lives. Implementing these strategies will help us preserve the aesthetic appeal and functionality of our belongings, ensuring they withstand the test of time.
Exploring Alternative Applications of Corroded Metal

Within the realm of oxidized metal lies a treasure trove of unconventional uses, where the rough texture, unique colors, and distinct properties of rusted iron are repurposed in a variety of interesting ways. Delving into the realm of corrosion can reveal innovative applications that extend far beyond the traditional confines of metalworking.
One striking avenue for exploring the potential of rusted iron is in the realm of art and design. The rustic and weathered appearance of corroded metal can add depth and character to sculptures, installations, and architectural elements. This unconventional approach to incorporating rust into artistic creations provides a distinct visual appeal and challenges traditional notions of beauty and aesthetics. From abstract sculptures to functional pieces of furniture, the allure of oxidized iron captures the imagination of artists, designers, and appreciators alike.
Another intriguing possibility lies in employing rusted iron in the field of interior design. The raw, earthy tones and rough textures of corroded metal can create a sense of warmth and authenticity in living spaces. Whether it's through accent pieces, light fixtures, or even entire feature walls, incorporating rusted iron elements into a home or commercial space can evoke a sense of history, craftsmanship, and a connection to natural processes. This unconventional use of corroded metal adds a touch of intrigue and uniqueness to any interior environment.
Rusted iron is not limited to the realm of art and design, as its distinctive qualities can be embraced in functional applications as well. The corrosion process can imbue iron with enhanced durability and resistance, making it a viable option for outdoor structures and installations. From fences and gates to outdoor furniture and decorative features, the weathered appearance of rusted iron can enhance the overall aesthetic while providing longevity and strength. Exploring these unconventional uses of corroded metal in functional settings can yield surprising and innovative results.
In summary, the exploration of unconventional uses for rusted iron reveals a vast and fascinating world beyond the traditional realm of metalworking. From art and design to interior spaces and functional applications, the distinctive qualities of corroded metal offer opportunities for creativity, visual appeal, and durability. By embracing the unconventional, a new dimension of possibilities unfolds, showcasing the versatility and allure of rusted iron in various contexts.
FAQ
What is rust iron?
Rust iron, also known as iron oxide, is a reddish-brown coating that forms on the surface of iron or steel when exposed to oxygen and moisture for an extended period of time.
Why does iron rust?
Iron rusts due to a chemical reaction called oxidation. When iron comes into contact with oxygen and water, it undergoes a process called corrosion, resulting in the formation of iron oxide or rust.
What causes oxidation of iron?
Oxidation of iron occurs when iron reacts with oxygen in the presence of moisture. The combination of oxygen, water, and the presence of certain salts or acids accelerates the oxidation process.
Is rust harmful?
Rust itself is not harmful to humans, but it can weaken the structural integrity of iron or steel objects. Additionally, if consumed or inhaled in large quantities, iron oxide can be harmful to health.
Can rust be prevented or removed?
Rust prevention can be achieved by applying protective coatings, such as paint or specialized rust inhibitors, to the surface of iron or steel. Rust can be removed using various methods, including abrasive techniques like sanding or using rust dissolving chemicals.
What causes iron to rust?
Iron rusts due to a chemical reaction called oxidation. When iron is exposed to oxygen and moisture, a process occurs where the iron atoms combine with oxygen molecules to form iron oxide, which is commonly known as rust.



