The quest for understanding the perplexing intricacies of nature has always fascinated scientists and researchers alike. One such enigma that continues to bewilder the scientific community revolves around the phenomenon of water fragmentation, an elusive process that intrigues both chemists and physicists. The ability to decipher the secrets of this mysterious occurrence holds immense potential for advancements in various fields, ranging from renewable energy to biological processes.
As we voyage into the depths of this captivating topic, we embark on a journey to unravel the enigma hidden within water fragmentation. While the facade of tranquility might lead one to believe that water is a homogeneous substance, scientific inquiry has revealed a hidden complexity. The process of water fragmentation involves the separation of water molecules into smaller units, each carrying its own unique properties and potentials.
The implications of understanding water fragmentation extend far beyond watery confines. By comprehending the underlying mechanisms that drive this process, scientists hope to unlock a wealth of possibilities. From catalyzing the production of sustainable energy sources, such as hydrogen, to gaining insight into the behavior of aqueous solutions in biological systems, the implications of unraveling this enduring puzzle are truly boundless.
The Fascinating Science behind Hydrogen and Oxygen Separation

In this section, we delve into the captivating scientific principles behind the intriguing phenomenon of water splitting, where hydrogen and oxygen are separated. Without directly addressing the specific topic of dreaming or the process of water splitting, we explore the underlying mechanisms that make this process possible.
Water splitting is a fascinating natural phenomenon that involves the separation of water molecules into their constituent elements: hydrogen and oxygen. This intricate process occurs through various methods, such as electrolysis or photosynthesis, each relying on specific scientific principles.
Electrolysis: Electrolysis, a well-established method for water splitting, utilizes an electrical current to split water into hydrogen and oxygen. By applying an external electric potential, water molecules undergo chemical reactions at the electrodes, resulting in the formation of hydrogen gas at the cathode and oxygen gas at the anode.
Photosynthesis: In contrast to electrolysis, photosynthesis is a naturally occurring process in plants that involves the conversion of light energy into chemical energy. During the light-dependent reactions of photosynthesis, water molecules are split, releasing oxygen gas and generating protons and electrons that eventually contribute to the production of energy-rich molecules such as carbohydrates.
The fundamental understanding of the science behind water splitting has significant implications in several fields, including renewable energy generation, storage, and environmental sustainability. Researchers aim to enhance the efficiency and scalability of water splitting methods to harness hydrogen as a clean and abundant energy source.
In this section, we have explored the captivating scientific principles behind water splitting, shedding light on how hydrogen and oxygen can be separated. By examining the methods of electrolysis and photosynthesis, we have unveiled the intricate mechanisms involved in this fascinating phenomenon. The fundamental knowledge derived from this research can pave the way towards a more sustainable future.
Unraveling the Potential of Harnessing Water Splitting for Sustainable Energy
In this section, we explore the inherent capabilities of water splitting as a promising avenue for achieving sustainable energy solutions. By delving into the unexplored realm of water splitting, we open the door to a future where clean and renewable energy sources prevail.
Water splitting, which involves the separation of water molecules into hydrogen and oxygen, holds immense potential in transforming our energy landscape. By harnessing this process, we can unlock a sustainable and abundant source of energy. This section aims to unravel the underlying mechanisms and possibilities associated with water splitting, highlighting its significance in terms of sustainability and environmental impact.
Hydrogen, being the primary product of water splitting, serves as an exceptional carrier of energy. Considered one of the most abundant elements in the universe, hydrogen possesses remarkable potential as a clean and sustainable fuel. It can be utilized in various applications, including transportation, electricity generation, and industrial processes, without emitting harmful greenhouse gases.
Moreover, water splitting can pave the way for oxygen production, further contributing to environmental sustainability. The production of oxygen from water splitting could support the development of life-sustaining technologies, such as improving air quality or enabling oxygen-dependent industries.
Unraveling the true potential of water splitting requires a deep understanding of the underlying catalysts and materials involved. Researchers worldwide are actively exploring novel catalysts and materials that can enhance the efficiency and stability of the water splitting process. These advances are crucial in making water splitting a financially viable and scalable option for sustainable energy generation.
In conclusion, by unraveling the vast potential of water splitting, we can pave the way towards a more sustainable and energy-efficient future. Through ongoing research and innovation, we can harness the power of water to meet our growing energy demands while minimizing the impact on our planet.
Exploring the Key Players in Enhancing Water Splitting Reaction
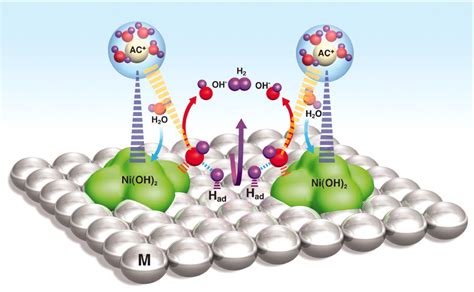
Introduction: In this section, we delve into the individuals and components that play a crucial role in amplifying the efficiency and efficacy of the water splitting reaction, a captivating scientific phenomenon that captivates researchers worldwide. By understanding the key players in this process, we can unlock the potential for sustainable energy production and explore innovative ways to utilize the power of water splitting for a greener future.
The Catalysts – Driving the Reaction: One of the primary elements in the water splitting reaction is the catalyst, which significantly influences the overall efficiency. Catalysts are substances that initiate and speed up chemical reactions without undergoing any change themselves. In the context of water splitting, catalysts facilitate the decomposition of water molecules into hydrogen and oxygen gases, providing a feasible route for harnessing clean energy. Exploring various catalysts and their unique properties is of utmost importance for unraveling the full potential of the water splitting reaction.
Photons – Energizing the Process: Another key player in water splitting is light energy. Photons, the fundamental units of light, are responsible for energizing the reaction and driving the splitting of water molecules. By absorbing incoming photons, the catalysts are activated, initiating the complex sequence of reactions that ultimately result in the separation of hydrogen and oxygen gases. Understanding the interaction between photons and catalysts is vital for devising strategies to maximize the efficiency of water splitting reactions.
The Elusive Role of Proton Transport: Proton transport, the movement of positively charged hydrogen ions, is an essential yet enigmatic aspect of the water splitting reaction. This process involves the transfer of protons from the water molecule to the catalyst, ultimately leading to the production of hydrogen gas. Unraveling the mechanisms and intricacies of proton transport is vital for enhancing the overall performance of water splitting reactions and improving the sustainability of hydrogen-based energy systems.
Conclusion: By exploring the key players in water splitting reactions, namely catalysts, photons, and proton transport, we can gain a deeper understanding of this intriguing scientific process. Investigating the properties and interactions of these components offers valuable insights into enhancing the efficiency, stability, and scalability of water splitting reactions. These advancements have the potential to revolutionize clean energy production and pave the way for a sustainable future fueled by the power of water splitting.
Exploring the Challenges and Breakthroughs in Harnessing Water Splitting for Renewable Energy
In the quest for sustainable energy sources, researchers have turned their attention to water splitting as a potential solution. This process involves breaking down water molecules into hydrogen and oxygen, which can be used as clean and efficient fuels. However, the road towards effectively harnessing water splitting as a renewable energy source is full of challenges and requires groundbreaking innovations in various aspects.
Advancing Catalysts: One of the primary challenges in water splitting lies in finding efficient catalysts that can facilitate the reaction without requiring excessive energy input. Researchers are exploring various materials including transition metal oxides, metal alloys, and molecular complexes to enhance the catalytic activity for both the hydrogen and oxygen evolution reactions.
Optimizing Energy Efficiency: Another significant hurdle in the quest for water splitting as a renewable energy source is improving energy efficiency. Researchers are working towards maximizing the efficiency of electrolysis, which is the process of using an electric current to split water. This involves optimizing cell designs, membrane materials, and reducing resistance encountered during the electrolysis process.
Overcoming Stability Issues: A critical breakthrough in harnessing water splitting as a renewable energy source is ensuring long-term stability of the catalysts and materials involved. The harsh conditions of the electrolysis process, including high temperatures and corrosive environments, pose challenges in maintaining catalyst activity over extended periods. Developing catalysts and materials with exceptional stability is a key area of research.
Integrating Renewable Energy Systems: Successful implementation of water splitting as a renewable energy source requires integration with existing renewable energy systems. Researchers are exploring ways to combine water splitting with other clean energy sources, such as solar or wind, to maximize energy efficiency and storage capabilities. This integrated approach will contribute to a more sustainable and reliable renewable energy infrastructure.
Overcoming the challenges in harnessing water splitting as a renewable energy source requires collaborative efforts from researchers, engineers, and policymakers. Breakthroughs in catalyst design, energy optimization, stability enhancement, and integration with the existing renewable energy systems hold the key to unlocking the full potential of water splitting as a sustainable energy solution.
Exploring New Frontiers in Advancing Water Splitting Technology
In this section, we will delve into the exciting prospects and advancements that lie ahead in the field of water splitting technology. By pushing the boundaries of scientific understanding and technological capabilities, researchers are opening up new horizons to harness the power of water as a renewable energy source.
One area of focus is the development of innovative catalyst materials. These substances play a crucial role in facilitating the splitting of water into hydrogen and oxygen gases. Scientists are tirelessly exploring various materials, such as metal oxides, alloys, and even artificial enzymes, in their quest to improve the efficiency and stability of water splitting reactions.
| Advancements in Catalyst Materials | Applications and Future Possibilities |
|---|---|
| Exploring new metal oxide catalysts with enhanced reactivity | Utilizing hydrogen as a clean energy source for transportation |
| Investigating the potential of alloy catalysts in achieving higher conversion rates | Integrating water splitting technology with renewable energy systems |
| Designing novel artificial enzyme catalysts for improved selectivity | Implementing large-scale water splitting for power generation |
Another exciting avenue for future advancements is the exploration of cutting-edge technologies, such as photoelectrochemical cells and photovoltaic devices, to drive water splitting reactions. By combining the principles of solar energy conversion with water-splitting processes, scientists aim to create efficient and sustainable methods of producing hydrogen fuel from sunlight.
Furthermore, ongoing research focuses on understanding and optimizing the different steps involved in water splitting. By unraveling the intricate mechanisms behind the catalyst-electrolyte interfaces, researchers aim to eliminate inefficiencies and develop strategies to minimize energy losses during the reaction.
This section will highlight the latest breakthroughs and research initiatives that hold immense promise in transforming water splitting technology. It serves as a glimpse into the exciting future where clean and abundant hydrogen fuel is harnessed through the advancements in this field.
FAQ
What is the mysterious phenomenon being unveiled in the article?
The mysterious phenomenon being unveiled in the article is the process of splitting water.
Why is splitting water considered a mysterious phenomenon?
Splitting water is considered a mysterious phenomenon because it involves separating water molecules into hydrogen and oxygen gases, which requires a significant amount of energy and specific catalysts.
What are the potential applications of splitting water?
The potential applications of splitting water include generating clean and renewable hydrogen fuel, which can be used to power cars, produce electricity, and serve as a substitute for fossil fuels, thus reducing greenhouse gas emissions and combating climate change.



