In the realm of metallurgy, there exists an extraordinary and enigmatic process, concealed behind a shroud of mystery. A metamorphosis that unveils itself as a hypnotic dance of elemental forces, leaving onlookers spellbound by its sheer magnificence. This captivating spectacle, free from the constraints of definition, holds in its fiery embrace the key to unlocking unlimited potential.
As this alchemical performance unfolds, the boundaries of reality seem to blur, ushering us into a realm where the sublime meets the tangible. It is a dance between delicate temperatures and transformative properties, where solid structures are coerced to surrender their rigidity, and the concept of permanence becomes but an illusion. The ever-changing nature of matter is laid bare, revealing the hidden depths of its true essence.
Within this mesmerizing display lies the heart of a clandestine journey, one that transcends human comprehension and touches upon the divine. As the elements of metal succumb to the allure of intense heat, they undergo a celestial evolution, shedding their solid form to embrace the liquid embrace of molten state. In this moment of transition, the very essence of metal undergoes an alchemical rebirth, transcending its original state to become a fluid embodiment of infinite possibilities.
It is in this molten cauldron where imagination merges with reality, where artisans harness the primal forces of nature. They become sculptors, shaping the amorphous mass through skill and creativity, transforming it into gleaming works of art or powerful tools that would shape the world around us. The molten metal becomes a vessel for human ingenuity, embodying the aspirations and dreams of those who dare to manipulate its fiery embrace.
The Scientific Explanation Behind the Transformation of Iron
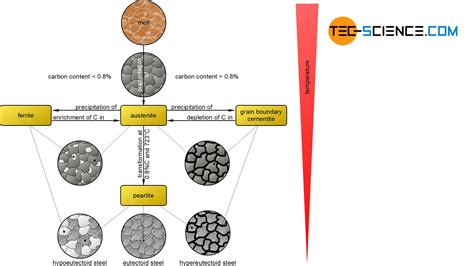
In this section, we delve into the fascinating science that lies behind the mysterious transformation of iron. By understanding the intricate processes involved in melting iron, we can gain insights into its properties and utilize this knowledge for various applications.
Iron melting is a complex phenomenon that involves the application of intense heat to convert solid iron into its molten state. The science behind this transformation lies in the behavior of iron atoms and their interactions with the surrounding environment. As heat is applied, the thermal energy causes the atoms to vibrate vigorously, overcoming the forces holding them in a solid lattice structure.
Upon reaching the iron's melting point, these vibrations become so intense that the bonds between the atoms are disrupted, and the solid structure begins to break down. At this critical temperature, known as the melting point, the iron undergoes a phase transition from a solid to a liquid state.
This process of iron melting is not just a simple change in physical state but involves a variety of scientific principles. The intermolecular forces holding the iron atoms together, such as metallic bonding and Van der Waals forces, play a significant role in determining the behavior of iron during the melting process.
Furthermore, the precise temperature at which iron melts depends on several factors, including its chemical composition and purity. Impurities present in the iron can alter its melting point, making it either higher or lower than the pure metal.
- Factors influencing the melting point of iron:
- Purity of the iron
- Chemical composition and impurities
- Pressure and atmospheric conditions
- Heating rate and method
Understanding the scientific principles behind iron melting enables researchers and engineers to control this transformation effectively. This knowledge has numerous applications in industries such as metallurgy, manufacturing, and engineering, where the ability to manipulate iron's physical state is essential for creating various products, structures, and materials.
As we continue to explore the intriguing mysteries of melting iron, we uncover new scientific insights that propel advancements in materials science and pave the way for innovation in various fields.
The Vital Role of Heat in the Transformation of Iron
In the realm of metallurgy, the process of melting iron brings forth an intriguing metamorphosis, governed by an indispensable force: heat. This section explores the profound impact that heat exerts on the transformation of iron, delving into its essential role in unlocking the latent potential and malleability of this remarkable metal.
Heat – a fundamental energy form that imbues iron with the ability to undergo a remarkable metamorphosis. As heat engulfs the solid iron, its individual particles begin to vibrate with increasing intensity, gaining energy and mobility. This kinetic energy disrupts the rigid lattice structure of the iron, gradually loosening the bonds between its atoms.
Temperature serves as a critical determinant in the process, as it defines the level of thermal energy within the system. By raising the temperature, we usher in a domino effect – the increased thermal energy prompts a progressive weakening of the iron's interatomic forces, paving the way for its eventual liquefaction.
Thermal expansion further accentuates this process, enabling the iron to undergo a volumetric expansion as heat infiltrates its crystalline structure. As an outcome of this expansion, the gaps between particles widen, permitting the mobility necessary for further transformation.
Moreover, the metamorphosis of iron under heat is not merely restricted to its physical state. The influence of heat extends to the chemical properties of iron as well. Elevated temperatures allow for increased reactivity, fostering the formation of unique chemical compounds and alloys.
To truly appreciate the mysterious transformation of iron melting, one must acknowledge the paramount role heat plays in enabling this metamorphosis. From its ability to disrupt the rigid lattice structure to initiating chemical reactions, heat stands as the catalyst that unlocks the latent potential of iron, ultimately shaping it into a malleable and versatile material.
Exploring Different Methods of Iron Fusion

In this section, we will delve into the various techniques employed in the fusion of iron, uncovering the diverse methods utilized to transform this durable metal.
Throughout history, mankind has developed numerous approaches to the fusion of iron, each with its own distinct advantages and limitations. From the ancient smelting techniques perfected by early civilizations to the cutting-edge technologies utilized in modern times, the endeavor to melt iron has been a fascinating journey of innovation and discovery.
1. Traditional Blast Furnace: One of the most widely recognized methods is the traditional blast furnace, which has been employed for centuries. This furnace utilizes a combination of heat, coke, and limestone to produce a chemical reaction that results in the melting of iron ore. The process involves a series of carefully controlled steps, ultimately leading to the extraction of molten iron.
2. Electric Arc Furnace: In contrast to the traditional blast furnace, the electric arc furnace provides a more modern and energy-efficient approach to iron fusion. This method employs the use of an electric arc, generated by powerful electrodes, to heat and melt the iron. It offers greater flexibility in terms of the types of iron that can be melted, as well as the ability to control the temperature more precisely.
3. Induction Furnace: Another method worth exploring is the induction furnace, which utilizes electromagnetic induction to generate heat for iron fusion. By placing the iron within a coil and passing alternating current through it, a magnetic field is created that induces heat, causing the iron to melt. This technique offers advantages such as faster melting times and the ability to easily regulate temperature levels.
4. Cupola Furnace: The cupola furnace, while less commonly used in modern times, played a significant role in iron melting during the industrial revolution. This tall and cylindrical furnace relies on the combustion of coke and air to generate intense heat, melting the iron in the process. Despite its inefficiency compared to more modern methods, the cupola furnace still holds historical significance in the evolution of iron melting techniques.
By exploring these different methods of iron fusion, we gain a deeper understanding of the science and artistry involved in transforming this seemingly indestructible material. Each approach brings its unique characteristics and advantages, contributing to the continued improvement and advancement of iron melting techniques.
The Influence of Extreme Temperatures on the Metamorphosis of Iron
Exploring the impact of high temperatures on the transformation of iron has been a subject of significant interest and study. The interaction between extreme heat and this versatile metal has long fascinated scientists and engineers alike. By subjecting iron to intense temperatures, researchers have uncovered a plethora of intriguing phenomena and discovered vital insights into its metamorphic behavior.
When exposed to excessive heat, iron goes through a remarkable process of alteration. The profound influence of elevated temperatures on Iron's structural properties and chemical composition has far-reaching implications in numerous industries. Understanding how extreme heat affects the transformation of iron is of paramount importance, as it allows for the development of improved industrial processes, enhanced material properties, and advancements in various scientific fields.
One consequence of subjecting iron to high temperatures is the alteration of its crystalline structure. As iron is heated, its atoms gain energy and start to vibrate more vigorously. This heightened motion leads to the breaking and rearranging of atomic bonds, resulting in a change in the overall crystal lattice structure. This transformation can lead to notable differences in the mechanical, electrical, and magnetic properties of the metal, making it a fascinating avenue for scientific exploration.
Another intriguing aspect of iron's response to extreme temperature conditions is the impact on its chemical composition. At elevated temperatures, iron can react with surrounding gases, forming various compounds and oxides. This chemical interaction, known as oxidation, can significantly alter the appearance, integrity, and functionality of the metal. By comprehending the intricacies of iron's reactions with different elements and gases at high temperatures, researchers can develop strategies to mitigate corrosion, preserve material integrity, and optimize its performance in diverse applications.
Overall, the exploration of how high temperatures influence the transformation of iron opens up new possibilities for industrial advancements and scientific breakthroughs. From enhanced manufacturing processes to the development of novel alloys, the knowledge gained from studying the metamorphosis of iron under extreme heat has the potential to revolutionize multiple industries. By delving deeper into this captivating phenomenon, researchers can unlock the mysterious secrets hidden within iron's response to extreme temperatures.
The Vital Role of Carbon in the Metamorphosis Process
Carbon plays a pivotal role in the remarkable transformation that occurs during the melting of various metals. Its significance lies in its ability to influence the metamorphosis of substances, acting as a catalyst for the molecular changes that take place. It is through the interplay of carbon atoms that the complex process of melting unfolds, allowing for the creation of new forms and structures.
Carbon's presence in the melting process is essential for several reasons:
1. Facilitating the breakdown of metallic bonds: By infiltrating the lattice structure of metals, carbon weakens the existing strong metallic bonds. This weakening of bonds enables the metal atoms to move more freely, ultimately leading to the liquefaction of the solid metal.
2. Promoting the removal of impurities: Carbon has a high affinity for various impurities, such as sulfur and oxygen, which may be present in metal alloys. During the melting process, carbon reacts with these impurities and forms volatile compounds that can be easily removed, resulting in a purer end product.
3. Adjusting the metal's properties: The inclusion of carbon in the melting process allows for the adjustment of the final metal's properties, such as hardness and strength. By controlling the carbon content, metallurgists can achieve desired material characteristics, making it adaptable for various applications.
4. Enabling the formation of new alloys: Carbon's ability to combine with other elements expands the possibilities for the creation of new alloys. These alloys can exhibit unique properties and characteristics, making them valuable in industries such as automotive manufacturing, aerospace engineering, and construction.
Thus, carbon's multifaceted role in the melting process is indispensable, as it not only facilitates the transformation of metals but also allows for the development of innovative materials with diverse applications. Understanding and harnessing the influence of carbon opens up a world of possibilities in the realm of metallurgy.
Unraveling the Chemistry Behind the Metamorphosis of Iron
Exploring the intricacies of the chemical changes that occur during the process of iron melting unveils a world of captivating transformations and fascinating reactions. Through delving into the understanding of these chemical phenomena, we gain profound insights into the science of metallurgy and the fundamental principles that govern the metamorphosis of iron.
As the heat embraces the solid iron, a series of captivating alterations begins to take place. At the molecular level, the bonds holding the iron atoms together begin to loosen, allowing for a gradual breakdown of the rigid structure. The thermal energy confers increased mobility to the iron atoms, creating a dynamic environment where they can interact with other elements and compounds, thus initiating a cascade of intriguing chemical reactions.
- Atomic rearrangements: The elevated temperature prompts the iron atoms to rearrange their positions in a dynamic and vibrant dance. This restructuring eventually leads to the formation of new crystal structures, each with its unique arrangement and properties.
- Oxidation reactions: The influx of heat introduces oxygen to the iron, instigating a complex series of oxidation reactions. Oxygen molecules eagerly bind with iron atoms, resulting in the formation of iron oxides. These oxides can vary in composition, giving rise to diverse forms of iron oxide compounds such as hematite, magnetite, and wustite.
- Impurity interactions: During the melting process, impurities present in the iron interact with the evolving chemistry. Some impurities may form compounds with iron, altering its properties, while others may segregate and concentrate at specific locations, influencing the overall quality of the final product.
Understanding the chemical changes that occur during iron melting serves as a stepping stone towards optimizing industrial processes and enhancing the properties and performance of iron-based materials. By shedding light on the mysteries behind this metamorphosis, we can unveil new possibilities for the practical applications of iron in various fields, from construction and infrastructure to manufacturing and engineering.
The Role of Fluxes in the Crucial Process of Iron Fusion
Within the captivating realm of iron fusion, a fascinating aspect that holds great significance is the utilization of fluxes. These essential substances play a pivotal role in facilitating the transformation of iron through their intriguing interactions and profound effects.
Fluxes, with their remarkable properties, act as catalysts in the process of iron fusion by altering the physical and chemical conditions within the melting furnace. They provide a crucial bridge between the solid state of iron and its molten form, effectively priming the metal for its metamorphosis.
Through the introduction of fluxes into the molten iron, unwanted impurities are diligently expelled, suspended particles are effectively dispersed, and the overall viscosity of the liquefied metal is optimized. This orchestrated dance of fluxes ensures the creation of a more refined iron alloy, ultimately enhancing its strength, durability, and structural integrity.
One of the key attributes of fluxes lies in their ability to lower the melting point of iron. By reducing the melting temperature, fluxes enable the iron to transition smoothly from its solid to liquid state. This lower temperature also serves to minimize energy consumption during the fusion process, rendering it more efficient and cost-effective.
Furthermore, fluxes not only aid in purifying the molten iron, but they also prevent the formation of undesirable compounds and enhance the alloying capabilities. They ingeniously act as reactive agents, removing harmful elements and fostering desirable chemical reactions, resulting in the production of high-quality iron with improved mechanical properties.
In conclusion, the significance of fluxes in the mesmerizing journey of iron fusion cannot be understated. Their ability to alter the physical and chemical characteristics of molten iron plays a crucial role in obtaining a superior and more versatile end product. By harnessing the power of fluxes, the realm of iron melting embraces a captivating transformation, unlocking the limitless potential of this remarkable metal.
Exploring the Physical Characteristics of Molten Iron
The Intriguing Study of Molten Iron's Physical Properties
Within the realm of materials science and metallurgy, researchers have long been captivated by the enigmatic nature of molten iron. In order to unravel its secrets, extensive investigations have been conducted to comprehensively examine the physical characteristics exhibited by this fascinating substance.
Temperature, viscosity, density, and electrical conductivity are among the primary attributes that define the behavior of molten iron. Understanding these properties is not only crucial for the iron industry but also provides valuable insights into other fields of science and engineering.
Temperature: One of the most striking aspects of molten iron is its temperature. At extreme heat levels, it transforms from its solid state into a mesmerizing liquid form. This transition is accompanied by significant changes in its physical properties, prompting the need for a detailed investigation into the behavior of iron at different temperatures.
Viscosity: Molten iron exhibits a unique range of viscosity that varies depending on temperature. Its ability to flow and resistance to deformation play a vital role in the industrial applications of iron. By understanding and manipulating its viscosity, scientists have made advancements in various fields, including metal casting and alloy production.
Density: The density of molten iron differs from its solid state due to changes in the arrangement of its atoms as temperature rises. This characteristic has significant implications for industrial processes such as steelmaking, as it affects the ease of separation and purification during the production of various iron-based alloys.
Electrical Conductivity: Molten iron possesses notable electrical conductivity properties. This attribute has significant implications for the design and optimization of electric arc furnaces, where the heat generated from passing electric current through molten iron plays a pivotal role in the production of commercial iron and steel products.
In conclusion, the exploration of the physical properties exhibited by molten iron offers invaluable insights into the behavior and potential applications of this extraordinary substance. The study of temperature, viscosity, density, and electrical conductivity provides a comprehensive understanding of molten iron, contributing to advancements in the iron industry and beyond.
Applications and Uses of Molten Iron in Various Industries

Molten iron, a substance formed through the process of heating and melting iron, holds great significance in numerous industries worldwide. With its exceptional properties and diverse applications, molten iron plays a crucial role in the advancement and innovation of various sectors.
The versatility of molten iron finds its application in the construction industry, where it serves as a fundamental material for creating robust structures such as bridges, skyscrapers, and highways. Its high strength and durability make it an ideal choice for engineers and architects as it ensures the stability and longevity of these vital infrastructure projects.
Furthermore, the automotive industry heavily relies on molten iron for the production of automobile components. Its excellent casting properties enable the manufacturing of complex shapes and intricate designs, allowing for the creation of intricate engine blocks, cylinder heads, and suspension components. The use of molten iron in this sector ensures the production of high-performance vehicles that meet both safety and efficiency standards.
In the manufacturing of appliances and machinery, molten iron displays its exceptional heat conductivity and thermal resistance. This makes it an indispensable element in the production of cookware, ovens, engines, and industrial equipment. The ability to withstand high temperatures and maintain thermal stability ensures the seamless functioning of these products, enhancing their overall performance and reliability.
| Industry | Applications of Molten Iron |
|---|---|
| Construction | Structural materials for bridges, skyscrapers, and highways |
| Automotive | Production of engine blocks, cylinder heads, and suspension components |
| Appliances | Manufacturing cookware, ovens, engines, and industrial equipment |
Moreover, the energy sector utilizes molten iron in power plants and renewable energy facilities. Its exceptional magnetism enables the generation and transmission of electrical power, making it an essential component in generators, transformers, and electrical machinery. By harnessing the magnetic properties of molten iron, the energy sector can produce a sustainable and reliable power supply.
In summary, the applications and uses of molten iron span across various industries, from construction to automotive, appliances to energy. Its versatile properties provide essential contributions to the development and progress of these sectors, ensuring the creation of robust structures, high-performance vehicles, durable appliances, and efficient energy generation systems.
FAQ
What is the article "Dreaming of the Mysterious Transformation: Melting Iron" about?
The article "Dreaming of the Mysterious Transformation: Melting Iron" explores the process of melting iron and delves into its fascinating properties and applications.
Why is melting iron considered a mysterious transformation?
Melting iron can be seen as a mysterious transformation because it undergoes a significant change in its physical state, turning from a solid to a liquid. This transformation involves complex physics and chemistry, making it intriguing and mysterious to many.
What are some of the applications of melted iron?
Melted iron has several applications across various industries. It is commonly used in the manufacturing of automobiles, machinery, and infrastructure materials like steel beams. Additionally, melted iron is also utilized in the production of household appliances and cooking utensils.



